This week, we meet thorium, a chemical element with the symbol Th and atomic number 90. This element is a bit unusual since it is the first element we've met that was named for a character in Norse mythology: it was named in honour of the Norse god of thunder (and war), Thor.
Swedish chemist Jöns Jakob Berzelius initially thought he had discovered this element in 1815 but soon realised that he had misidentified yttrium phosphate as being a new element, thorium. Berzelius apparently liked the name "thorium", since he reused it in 1829 when he was given a newly discovered mineral by Norwegian mineralogist Morten Thrane Esmark. It was from this rock that Berzelius extracted and identified thorium.
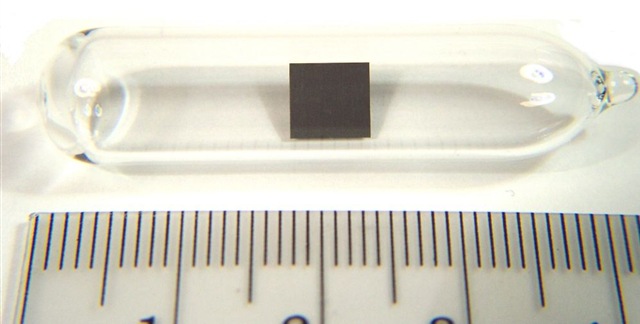
Even though we are working our way through the actinoid/actinide series, which is filled with what I think of as "invisible" elements, thorium is nice because it actually has a physical -- therefore photographable -- presence that I can share with you (refer to the above photograph).
Besides being photographable, there is something else to love about thorium: it gives us the chance to learn a useful new adjective, pyrophoric, which comes from the Greek for "fire-bearing". Pure thorium has the delightful quality of spontaneously igniting into flames when exposed to air.
Thorium is the most common actinoid and is surprisingly common in the wild, occurring in small amounts in most rocks and soils in the Earth's crust. For example, thorium is 200 times more abundant in the Earth's crust than silver, three times more abundant than uranium, and it is roughly as abundant as lead.
Pure thorium is a soft, lustrous silvery-white metal. If it doesn't burst into flames first, thorium will slowly tarnish when exposed to air, becoming gray, as you see above, and then finally black in colour. Thorium is very ductile and, like all actinoids, thorium is radioactive. Although 27 isotopes have been identified with half-lives ranging from less than ten minutes to 75,380 years, nearly all naturally-occurring thorium is thorium-232, which has a half-life of more than 14.05 billion years. Thorium is a weak alpha emitter, eventually decaying into lead-208.
Due to its radioactivity, thorium doesn't have many uses these days, but thorium oxide was used in the past for gas lighting in cities because it emits a dazzling blue-white light.
As a biologist, I have to admit that thorium isn't very interesting to us because it is not essential to life. In fact, the opposite is true: thorium is quite damaging to life, causing a variety of cancers including leukæmias, bone cancers, cirrhosis and cancers of the liver, and chromosomal abnormalities.
Considering what we know about thorium, it found some rather surprising -- and alarming -- uses in the past. For example, it was one of the ingredients in a toothpaste manufactured by the Berlin Auergesellschaft (Auer Company) in the early 1900s that was sold in Germany for many years. During the World War II, another quack toothpaste, Doramad Radioaktive Zahncreme, used thorium as an ingredient, falsely claiming that the "radiation increases the defenses of teeth and gums", and that "the cells are loaded with new life energy," whilst at the same time, "bacteria are [magically] hindered in their destroying effect."
Weirdly, this fixation with thorium-based toothpastes continued even after the conclusion of World War II when the Allies discovered a large stockpile of thorium held by a German military contractor. The Allies suspected this was intended for use in atomic weapons but later learned it was meant to be used in ... yep, you guessed correctly ... toothpaste.